Companies are using advanced technologies to manufacture their products, create intellectual …
Technology Can Make Return-to-work More Effective and Efficient
You are all probably aware of many of the standard strategies that can be employed to ensure timely …
Technology Can Make Return-to-work More Effective and EfficientRead More
Quality Job Profiles Are Critical to Optimally Manage Workers’ Compensation
First, let’s get our terminology straight. The terms “job profile” or “job description” are often …
Quality Job Profiles Are Critical to Optimally Manage Workers’ CompensationRead More
Save Time & Money By NOT Using an Independent Medical Exam
In the course of claim and medical management in workers’ compensation, adjusters can and should …
Save Time & Money By NOT Using an Independent Medical ExamRead More
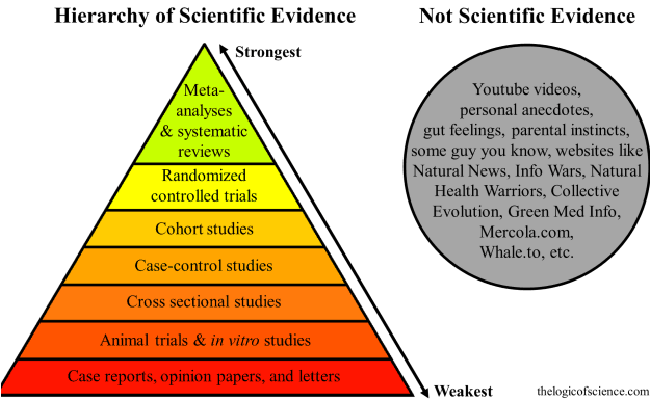
How Evidence Based Medicine & Clinical Guidelines Impact Workers’ Comp
Evidence-based medicine (EBM) is the conscientious, explicit, and judicious use of current best …
How Evidence Based Medicine & Clinical Guidelines Impact Workers’ CompRead More
Broadspire Medigram: Revisiting Some Controversial Topics
By Jacob Lazarovic, MD, FAAFP Senior Vice President and Chief Medical Officer, …
Broadspire Medigram: Revisiting Some Controversial TopicsRead More
Medigram: Measles And More
By Jacob Lazarovic, MD, FAAFP Senior Vice President and Chief Medical Officer, …
Accountable Care and Workers Compensation: Are They Compatible?
By Jacob Lazarovic, MD, FAAFP Senior Vice President and Chief Medical Officer, Broadspire First …
Accountable Care and Workers Compensation: Are They Compatible?Read More
Chronic Pain Management Matters
In 2006, The Center for Disease Control and Prevention (CDC) released its 30th annual report …
Ask Dr. Jake about H1N1 Planning, Testing and Guidelines in the Workplace
Some people follow Dr. Oz, but we follow Dr. Jake, Medical Director at Broadspire... who has great …
Ask Dr. Jake about H1N1 Planning, Testing and Guidelines in the WorkplaceRead More